Abstract
Introduction: Clinical trials are essential to establish the efficacy and safety of medical products; however, clinical trials may not always be representative of or generalizable to the intended patient population. Notably, patients with comorbidities, older adults, and patients from racial and ethnic groups are often under-represented in clinical trials for acute myeloid leukemia (AML) (Bierenbaum et al, Leuk Res, 2012). Real-world data (RWD) can provide insight into the utilization, effectiveness, and safety of treatment regimens in routine clinical practice and has demonstrated usefulness in understanding the current treatment landscape. The objective of this study was to describe patient characteristics and treatment patterns in the first-line setting among a real-world cohort of adult patients with ND-AML in the US.
Methods: This retrospective observational study used the COTA real-world database, a de-identified source of longitudinal electronic health record (EHR) data pertaining to the diagnosis, clinical management, and outcomes of patients with cancer. The study population included adult patients (≥ 18 years) diagnosed with ND-AML between January 1st, 2013 - December 31st, 2021. Patients were excluded if key study data were missing (i.e., date of initial AML diagnosis or date of outcome or last visit). First-line treatment regimens were mapped to relevant therapeutic categories (e.g., intensive chemo [IC] w/ cytarabine, hypomethylating agent with venetoclax [HMA+ven]) and summarized across clinical and demographic patient characteristics using descriptive analyses.
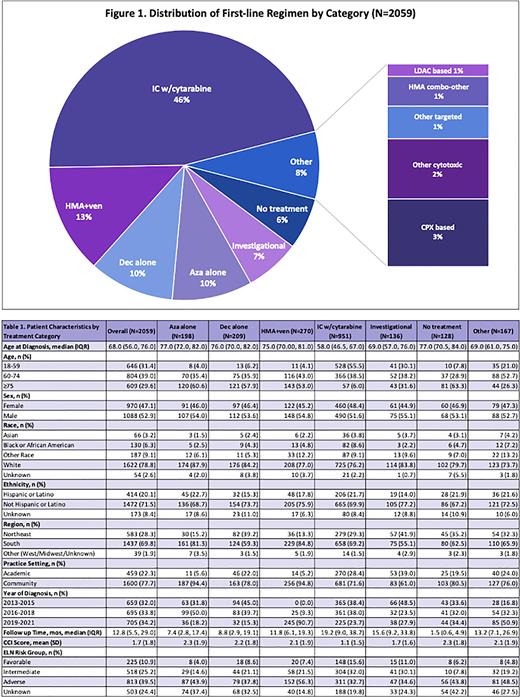
Results: Among a total of 2059 patients that met study criteria, distribution of first-line treatment by category is shown in Figure 1. The most common first-line treatments were IC w/ cytarabine (46.2%), followed by HMA+ven (13.1%), and decitabine (Dec) alone and azacitidine (Aza) alone (10.2% and 9.6%, respectively). Of the 951 patients who received IC w/cytarabine, 34 (3.6%) received add-on therapy with midostaurin and 26 (2.7%) received add-on therapy with gemtuzumab ozogamicin. Approximately 6.6% of the study cohort received an investigational product as part of a clinical trial as first-line therapy. Demographic and clinical characteristics and follow-up by treatment category are shown in Table 1. Relative to other regimens, a greater proportion of patients receiving upfront intensive chemotherapy were younger, had fewer observed comorbidities, and had a favorable European Leukemia Net (ELN) risk score. No clear differences in treatment patterns were seen by racial or ethnic group, other than potential underrepresentation of black/African American and Latino patients among those receiving investigational therapy. The proportion of patients treated with HMA+ven has increased over time starting in 2018, particularly in patients with ELN adverse risk, and the use of HMA alone decreased during the same time period. There was a potential decrease in use of IC w/cytarabine and a rise in use of HMA+ven during 2020. Use of single agent targeted therapies and LDAC-based therapies were relatively uncommon.
Conclusions: In our cohort of patients with ND-AML, the predominant first-line treatment was an intensive chemotherapy regimen. Treatment group by year of diagnosis indicates that over time, greater proportions of patients are receiving HMA+ven as first-line intervention, predominantly among older patients and those with adverse risk per ELN stratification. This study demonstrates the capability of using RWD to characterize the ND-AML treatment landscape, particularly after the introduction of novel therapeutic agents. Findings are subject to the inherent limitations associated with observational data, including the challenges in collecting and analyzing real-world data (e.g. missing treatment data or other characteristics) which may result in bias or limited generalizability of study findings. Future research may evaluate real-world safety or effectiveness, particularly in populations underrepresented in clinical trials, to inform the post-approval therapeutic landscape.
Disclosures
Fernandes:COTA, Inc: Current Employment. Wynne:Carisma Thereapeutics: Patents & Royalties: Patent relating to HIV therapy. Barcellos:COTA, Inc: Current Employment. Belli:COTA, Inc: Current Employment, Current equity holder in private company. Hansen:COTA, Inc: Current Employment, Current equity holder in private company. Wang:COTA, Inc: Current Employment, Current equity holder in private company.
Author notes
Asterisk with author names denotes non-ASH members.